|
It happens that there is an electrochemical solution to this
electrochemical problem.
Iron will not rust or oxidize if it is plated with a more
reactive metal-one with a more negative reduction
potential.
Aluminum is a possible candidate. If iron and aluminum are
in contact, iron will behave as the cathode and aluminum as
the anode, as their reduction potentials indicate:
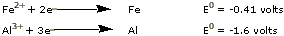
A coating of aluminum will prevent iron from being oxidized,
and its own oxide will protect the aluminum from continual
destructive corrosion.
But if you are going to aluminum-plate iron, you might as
well make the objects out of aluminum to begin with, and gain
the advantage of lightness.
Unfortunately, aluminum is expensive. A cheaper alternative
is to galvanize
the iron by giving it a thin coating of zinc.
|