|
The corrosion
of metals is an oxidation
process. Iron can be oxidized either by oxygen or by
acid, if enough moisture is present to allow ionic reactions
to proceed at an appreciable rate:
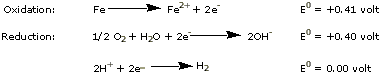
When iron rusts, metallic iron is oxidized first to the
+2 state and deposited as flakes of
Fe(OH)2 and FeO,
later being oxidized even further to
Fe(III). Aluminum corrodes even more vigorously,
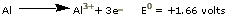
but the A1203 oxide coating, having
a crystal structure similar to the metal, adheres
tightly to the metal surface and prevents further corrosion.
In contrast, the crystal structures of metallic iron and iron
oxide are not similar, and the
two do not adhere.
The oxide flakes away as it forms, exposing
fresh metal for attack by oxygen or acid. A good layer
of paint adheres better than FeO, but still is not permanent.
|