|
Oxidations and reductions
involve the escape of electrons
from molecules or ions of one substance and their capture
by other chemical substances. As with the movement of entire
molecules discussed in previous sections, free
energy is the key to understanding this escaping tendency.
Oxidation-reduction (redox)
reactions are important because they are the principal sources
of energy on this planet, both natural or biological and artificial.
Oxidation of molecules by removal of hydrogen or combination
with oxygen normally liberates large quantities of energy.
The synthesis of reduced organic molecules (sugars) by photosynthetic
green plants is the main device for trapping and storing solar
energy on this planet.
Oxidation either can involve
the outright loss of electrons:
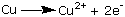
or the shifting away of
bonding electrons toward a more electronegative atom:
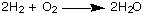
|