|
5. If 1 erg = 2.390 X 10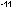 kcal, what is the energy in kilocalories of one mole of photons
of wavelength 6600
kcal, what is the energy in kilocalories of one mole of photons
of wavelength 6600 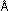 ? ?
6. If the energy of one photon is given by E = h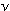 ,
then the energy of a mole of photons, a chemically useful piece
of information, is given by E = Nh ,
then the energy of a mole of photons, a chemically useful piece
of information, is given by E = Nh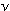 ,
in which N is Avogadro's number. From the conversion factors given
in Problems 3 through 5, calculate Nh in kcal ,
in which N is Avogadro's number. From the conversion factors given
in Problems 3 through 5, calculate Nh in kcal 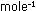 sec. Use this number directly with the frequency of 6600-
sec. Use this number directly with the frequency of 6600-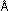 light to calculate the energy per mole of photons, and verify that
the answer is the same as for Problem 5.
light to calculate the energy per mole of photons, and verify that
the answer is the same as for Problem 5.
7. An even easier way of converting wavelength to energy
per mole of photons is:
 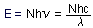
Calculate the constant Nhc in units of kcal 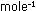 cm and kcal
cm and kcal 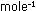
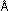 . (Your answer'should be Nhc = 286,000 kcal
. (Your answer'should be Nhc = 286,000 kcal 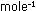
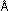 .) ,Use this to verify again the energy in kilocalories of a mole
of 6600-
.) ,Use this to verify again the energy in kilocalories of a mole
of 6600-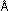 photons.
photons.
|
|