|
Davisson and Germer at Bell Telephone Laboratories tested this
idea, in 1927, by passing a thin beam of accelerated electrons through
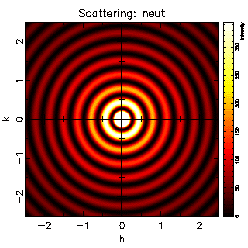
a metal foil. The pattern that they obtained, like that shown on
page 21, obviously is a pattern produced by diffraction of some
kind of waves by the metal atoms.
Knowing the wavelength of the x rays used, a physicist can measure
the radii of the diffraction rings in an x-ray pattern, such as
that shown on the last page, and calculate the spacings between
atoms in the metal.
With this information, he then can work backward from the radii
of the corresponding rings in the electron-diffraction pattern to
calculate the effective wavelength of the beam of electrons. It
was found that the wavelength of a beam of electrons depends on
the speed of the electrons. The faster the electrons the shorter
their wavelength. Electrons accelerated through a potential of 40,000
volts, or given a kinetic energy of 40,000 electron volts, have
a wavelength of 0.06 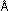 . .
|

|

