|
32.
How many bonding electrons, and how many and what kind of AO's,
remain unused in benzene after the o-hond skeleton is built? How
are these electrons and AO's used for further bonding in benzene?
How does this resemble the situation in ethylene and acety lene,
and in what important way is it different?
33. How would the electrons and AO's not involved in the
s-bond skeleton be used in the Kekule
bond model for benzene? In a perspective view of the hexagonal C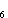 ring, sketch the bonding p MO's.
ring, sketch the bonding p MO's.
34. Repeat the preceding question for one of the Dewar models
of benzene.
35. In a similar perspective view of the benzene ring, sketch
the actual 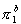 MO,
showing the rings of electron probability above and below the C MO,
showing the rings of electron probability above and below the C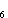 benzene-ring plane.
benzene-ring plane.
36. For all of the p MO's in
benzene, what is the electron probability density at the carbon
and hydrogen atoms? How does this compare with the density at the
C and H atoms for the p MO in ethylene?
(See Question 28.)
37. In what sense does bonding in the benzene molecule represent
a step backward from two-atom bonds to full-molecule orbitals? What
is this called? What effect on the energy levels of a molecule accompanies
such behavior? Give examples of inorganic oxygencontaining compounds
that show the same behavior.
38. In what sense is it possible to think of the actual bonding
in benzene as being a "mixture" of Kekule and Dewar structures?
What are these structures called? Is any actual alternation back
and forth between structures implied?
|
|