|
20.
Sketch the use of AO's in bonding in the water molecule, assuming
that unhybridized s and three p orbitals of oxygen are used. What
prediction does this model make about the H-0-H bond angle?
21. Sketch the use of AO's in bonding in the water molecule,
assuming that hybrid sp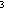 AO's are the starting point for the oxygen atom. What does this
predict about the H-0-H bond angle? What is the observed bond angle,
and what does this tell us about the relative merits of the bond
models of this question and the preceding one?
AO's are the starting point for the oxygen atom. What does this
predict about the H-0-H bond angle? What is the observed bond angle,
and what does this tell us about the relative merits of the bond
models of this question and the preceding one?
22. What physical explanation can be given for the decrease
of the H-0-H bond angle in water from its ideal tetrahedral value?
it explanation? How does this compare with the VSEPR explanation?
23. When an sp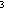 hybrid AO on carbon interacts with a hydrogen 1s orbital, what is
the symmetry of the resulting MO's? Why are the antibonding MO's
from such combinations ordinarily ignored?
hybrid AO on carbon interacts with a hydrogen 1s orbital, what is
the symmetry of the resulting MO's? Why are the antibonding MO's
from such combinations ordinarily ignored?
24. There is essentially free rotation about the carbon-carbon
bond in ethane, although the "staggered" conformation, with hydro
gens on one carbon rotated 60 away
from those on the other carbon, is 3 kcal mole away
from those on the other carbon, is 3 kcal mole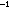 more stable than the "eclipsed" conformation, in which the hydrogens
on opposite carbon atoms overlap in a view down the carbon-carbon
bond axis. Why should this energy difference, small as it is, exist?
more stable than the "eclipsed" conformation, in which the hydrogens
on opposite carbon atoms overlap in a view down the carbon-carbon
bond axis. Why should this energy difference, small as it is, exist?
25. Sketch the bonding in ethylene, assuming sp2 hybridization
around the carbon atoms. In what sense would the bonding between
carbons be a double bond? What would this model predict for the
H-C-H bond angle at each end of the molecule?
|
|