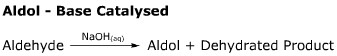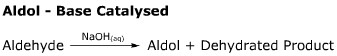
- This reaction features a rare example of an OH- leaving group.
- One can perform crossed aldol reactions on malonates without using a strong base to form an enolate. This reaction is difficult to stop however.
- Firstly the aldol is formed, then reaction proceeds to show the formation of the dehydrated product.
- The final stage of this reaction (formation of the dehydrated product) occurs via the E1cB
mechanism.
- This is a more controlled aldol reaction than its acid
catalysed alternative.
- This reaction can occur intramolecularly,
within a single molecule.
References:
- White
J.D.; Blakemore P.R.; Green N.J.; et al., J. Org. Chem., 2002, Volume 67,
No. 22, 7750-7760
- Wei
H.-X.; Hu J.; Purkiss D. W.; Paré P. W.; Tetrahedron Lett.,
2003, Volume 44, Issue 5, 949