Magnetic Moments
and Unpaired Electrons
Paramagnetic species, i.e. those with unpaired electrons,
are attracted by a magnetic field. We can measure the strength of
the attraction, and thereby calculate the magnetic moment of the
sample; and from the latter we can infer the number of unpaired
electron spins present.
The magnetic moment m is approximately related to the number
of unpaired electrons n by the formula:
m = [n(n + 2)]1/2 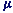 B
B
Where 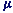 B is the Bohr magneton, the atomic unit of magnetic moment.
B is the Bohr magneton, the atomic unit of magnetic moment.
Derivation of this formula � Return to the menu