|
One of the consequences of the second law and the studies by people such as Kelvin and Clausius was the invention of a new and useful function, the entropy, S. As originally defined, entropy was strictly a function of heat and temperature. With enough experimental ingenuity and patience, the entropy of any substance at a given temperature can be calculated from calorimetric measurements. Because of the third law of thermodynamics, which states that the entropy of every pure, crystalline substance at absolute zero is zero, these values calculated from calorimetric measurements are called third-law entropies. They are tabulated as S0298 for elements and compounds in their standard states at 298�K, alongside heats of formation, in the appendix (and in any standard chemical handbook).
|
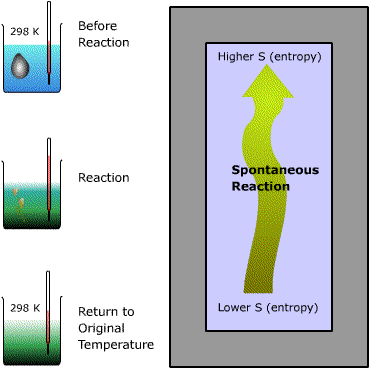 |