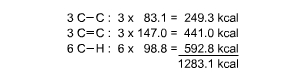|
One
can calculate heats of formation from bond-energy values for hundreds
of molecules, and never be in error more than a kilocalorie or two.
However, in those cases where the discrepancy is large, one can
learn something new about the nature of chemical bonding. Benzene
is a good illustration of this.
Let us try to calculate the standard heat of formation of benzene,
C6H6.
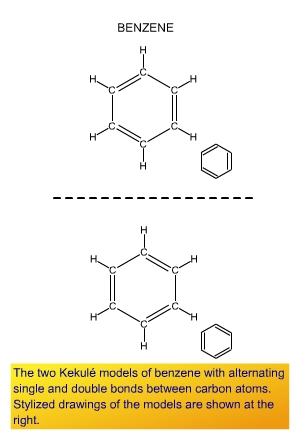
As we saw in Chapter 9, benzene is an example of a molecule for
which simple single bond and double bond ideas are inadequate, and
structures using them, such as the Kekul� structures at the right,
are wrong. Benzene has six electrons delocalized around the ring.
From a bond-energy viewpoint, how bad is the localized Kekul� model?
If we provisionally accept the Kekul� structures, then benzene has
three C-C single bonds, three C=C double bonds, and six C-H single
bonds. The energy involved in taking one mole of benzene molecules
apart into atoms is
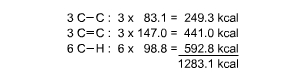
|
|