|
Concentrations usually are expressed in moles per liter for solutions,
or in mole fractions or partial pressures for gases. The equilibrium
constants expressed in partial pressures and moles per liter often
are designated by 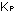 and
and 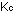 , respectively.
In general, , respectively.
In general, 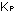 and
and 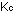 will have
different numerical values, but one can be calculated from the other
using the ideal gas law. will have
different numerical values, but one can be calculated from the other
using the ideal gas law.
Le Chatelier's principle is an important shortcut in predicting
how a system at equilibrium will behave. It says that if an outside
stress is applied to a chemical system at equilibrium, the system
will shift in such a way to reduce that stress.
If the stress is a pressure increase, the reaction will shift in
the direction that decreases the number of moles of reactants and
products. If the stress is a dilution, then equilibrium shifts to
increase the number of moles in the reaction mixture.
These adjustments do not change the numerical value of  .
If the temperature is raised, however, the reaction will shift in
the direction in which heat is absorbed, and .
If the temperature is raised, however, the reaction will shift in
the direction in which heat is absorbed, and  will change.
will change.
|
|
The equilibrium constant gives the ratio of products to reactants
at equilibrium. If, for a given set of experimental conditions,
the actual ratio
 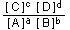
is less than  ,
equilibrium has not yet been reached, and will not be reached until
A and B have formed more C and D. Conversely, if the concentration
ratio is numerically larger than ,
equilibrium has not yet been reached, and will not be reached until
A and B have formed more C and D. Conversely, if the concentration
ratio is numerically larger than  ,
then the reaction is on the far side of equilibrium. Equilibrium
then will be attained only after C and D have broken up spontaneously
into more A and B. ,
then the reaction is on the far side of equilibrium. Equilibrium
then will be attained only after C and D have broken up spontaneously
into more A and B.
|