|
The concentration or pressure ratio
 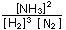
would decrease by a factor of (2)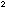 / (2)
/ (2) (2) = 1/4, and would become smaller than the equilibrium constant,
(2) = 1/4, and would become smaller than the equilibrium constant,
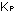 .
More .
More 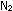 and
and 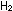 would react until the imbalance was corrected and the concentration
ratio again was equal to
would react until the imbalance was corrected and the concentration
ratio again was equal to 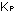 .
Pressure changes have no effect if the total number of moles of
gas is unchanged during the reaction. The HCl synthesis would be
unaffected by an increase or decrease in external pressure. .
Pressure changes have no effect if the total number of moles of
gas is unchanged during the reaction. The HCl synthesis would be
unaffected by an increase or decrease in external pressure.
These pressure effects are one example of an important general principle,
which, if understood, can save a lot of mathematics. This is Le
Chatelier's principle:
If external stress is applied to a system at equilibrium,
the equilibrium conditions will shift so as to compensate for, or
counteract, the stress.
 Right:
Henri Louis Le Chatelier (1850 - 1936) Right:
Henri Louis Le Chatelier (1850 - 1936)
|

|
