|
Pressure can be used to shift the
actual equilibrium conditions of any reaction during which the total
number of moles of gas changes. In the ammonia reaction three moles
of 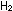 and
one of and
one of 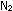 react to form two moles of ammonia. Increasing the pressure shifts
the equilibrium in the direction of more ammonia formation, and
decreasing the pressure leads to more dissociation of ammonia (see
above). The value of the equilibrium constant does not change,
react to form two moles of ammonia. Increasing the pressure shifts
the equilibrium in the direction of more ammonia formation, and
decreasing the pressure leads to more dissociation of ammonia (see
above). The value of the equilibrium constant does not change,
|
|
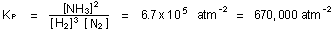
but the relative amounts of 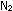 , ,
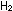 , and , and
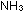 do change.
You can understand this by imagining that the total pressure on
the reaction vessel is doubled, so that the partial pressures of
each of the gases also are doubled. do change.
You can understand this by imagining that the total pressure on
the reaction vessel is doubled, so that the partial pressures of
each of the gases also are doubled.
|