|
Hydrolysis of natural fats with a base (usually NaOH or KOH) is
called saponification - literally, "soap making":
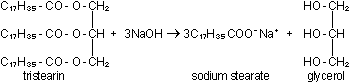
One difficulty with natural soaps is that their calcium and magnesium
salts are insoluble. If soap is added to hard water containing 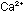 and
and 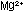 ,
a greasy soap scum of calcium and magnesium stearate results. One
solution to the problem is to use so much soap that all of the divalent
cations are precipitated as scum, and more soap is left for cleaning
purposes. This is messy and wasteful. Another solution is to remove
the divalent cations ahead of time, and to replace them by ,
a greasy soap scum of calcium and magnesium stearate results. One
solution to the problem is to use so much soap that all of the divalent
cations are precipitated as scum, and more soap is left for cleaning
purposes. This is messy and wasteful. Another solution is to remove
the divalent cations ahead of time, and to replace them by 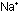 via and ion-exchange resin in a water-softener. A third possibility
is to use artificial detergents whose calcium and magnesium salts
are soluble.
via and ion-exchange resin in a water-softener. A third possibility
is to use artificial detergents whose calcium and magnesium salts
are soluble.
|
|
Sodium lauryl sulphate
is one such detergent:
 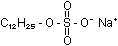
The trouble with many of these compounds is that they are not biodegradable,
and eventually will pollute water supplies. The carboxylic acids
are "natural" in the sense that they can be used as food
by a host of bacteria, and are broken down eventually to 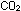 and
and 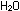 , to
blend into the environment. Never having been faced with hydrocarbon
sulfates prior tot he coming of man, bacteria have not evolved the
machinery to use such compounds. They remain untouched in water
and soil, occasionally leading to such monstrosities as rivers covered
with detergent foam. Biodegradable detergents have been developed
recently, which are not precipitated by calcium and magnesium ions,
yet which can be eaten by bacteria. , to
blend into the environment. Never having been faced with hydrocarbon
sulfates prior tot he coming of man, bacteria have not evolved the
machinery to use such compounds. They remain untouched in water
and soil, occasionally leading to such monstrosities as rivers covered
with detergent foam. Biodegradable detergents have been developed
recently, which are not precipitated by calcium and magnesium ions,
yet which can be eaten by bacteria.
|