|
Fats
are triesters of fatty acids and glycerol:
 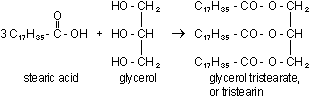
Tristearin is the most common animal fat, used for energy storage,
protection against physical damage, and heat insulation. We will
discuss tristearin and other fats and related compounds again in
Chapter 21.
The reverse of esterification is hydrolysis. Hydrolysis of
an ester was used as an example of an acid- or base-catalysed reaction
in Chapter 16. Without the help of an acid or base, the reaction
is slow becasue no easy path for hysrolysis exists. The reaction
liberates acid,
 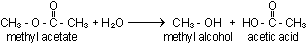
and therefore is pushed to completion by a base, which neutralizes
and removes the acid as fast as it is formed.
|
|