|
9. In metallic iron, the atoms are arranged at the corners and the
center of a cube of edge 2.866 Å, in the same body-centered
cubic structure shown in this chapter for lithium. What is the volume
of this unit cube, and how many iron atoms are there per unit volume
of this size? (Be careful. If an iron atom is shared between four
unit volumes, then only one quarter of the atom can be counted for
a given unit volume. What is the situation with regard to sharing
for one of the atoms at the corners of the cube? What about the
atom in the center of the cube?)
10. What is the atomic volume for iron, in Å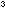 per atom? What is the atomic density, in atoms per Å
per atom? What is the atomic density, in atoms per Å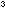 ?
How many atoms are there per cubic centimeter in iron? ?
How many atoms are there per cubic centimeter in iron?
11. The measured density of iron is 7.86 g cm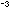 .
If the atomic weight of iron is 55.85, how many moles of iron are
there per cubic centimeter? .
If the atomic weight of iron is 55.85, how many moles of iron are
there per cubic centimeter?
12. Use the answers to Problems 10 and 11 to obtain an experimental
value for Avogadro's number. How does it compare with the value
that we have used before?
|
|
13. The structure of crystalline sodium chloride can be thought
of as being built from cubes with 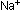 and
and 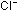 ions on alternating corners, as in the diagrams seen previously
in this chapter. The distance between the centers of
ions on alternating corners, as in the diagrams seen previously
in this chapter. The distance between the centers of 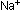 and
and 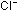 ions along an edge of the cube is 2.820 Å. (These crystal
spacings can be obtained from x-ray diffraction experiments.) How
many NaCl units are there in a cube 2.820 Å on a side? (Remember
the sharing of ions with several adjacent cubes. You should come
up with an answer of one half unit of NaCl per cube of edge 2.820
Å.) What is the density of sodium chloride, in NaCl pairs
per Å
ions along an edge of the cube is 2.820 Å. (These crystal
spacings can be obtained from x-ray diffraction experiments.) How
many NaCl units are there in a cube 2.820 Å on a side? (Remember
the sharing of ions with several adjacent cubes. You should come
up with an answer of one half unit of NaCl per cube of edge 2.820
Å.) What is the density of sodium chloride, in NaCl pairs
per Å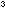 ?
What is the density in NaCl pairs per cm ?
What is the density in NaCl pairs per cm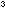 ? ?
14. The measured density of rock salt is 2.165 g cm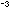 .
Calculate the molecular weight of NaCl from the atomic weights on
the inside back cover, and calculate the density of NaCl in .
Calculate the molecular weight of NaCl from the atomic weights on
the inside back cover, and calculate the density of NaCl in
moles cm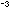 . .
15. Use your answers to Problems 13 and 14 to calculate Avogadro's
number. How does it compare with the value we have been using?
|