|
1 . What are the molecular weights of lithium hydroxide and hydrogen
fluoride?
2. In the neutralization of lithium hydroxide solution by hydrofluoric
acid solution, how many moles of HF are required for each mole of
LiOH?
3. How many moles of HF will be required to neutralize 100 g of
LiOH? How many grams of HF will this correspond to?
4. If the neutralized solution of the preceding problem is evaporated
to dryness, how many moles of crystalline lithium fluoride will
result? How many grams of LiF will there be?
5. How much water will be produced during neutralization of
100 g of LiOH? How does the total weight of lithium hydroxide plus
hydrogen fluoride starting materials compare with the weight of
water plus LiF at the end of the experiment?
|
|
6. If, in the lunar excursion module propulsion system, one molecule
of 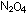 reacts with two molecules of hydrazine to yield three molecules
of
reacts with two molecules of hydrazine to yield three molecules
of 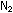 and four molecules of
and four molecules of 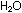 ,
write an equation for this reaction. Check your result against the
equation given previously in this chapter. Count the numbers of
atoms of N, H, and O on both sides of the equation. Are they the
same for each kind of atom? If this is so, then the equation is
said to be "balanced." ,
write an equation for this reaction. Check your result against the
equation given previously in this chapter. Count the numbers of
atoms of N, H, and O on both sides of the equation. Are they the
same for each kind of atom? If this is so, then the equation is
said to be "balanced."
7. Calculate the molecular weights of 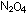 and hydrazine. How many moles of hydrazine would be required to
react with one mole of nitrogen tetroxide?
and hydrazine. How many moles of hydrazine would be required to
react with one mole of nitrogen tetroxide?
8. How many moles of hydrazine will be required to react with 500
g of 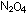 ?
How many grams of hydrazine will this be? How many grams of ?
How many grams of hydrazine will this be? How many grams of 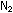 and water vapor will be ejected through the rocket nozzle? How does
this compare with the original weight of hydrazine and nitrogen
tetroxide?
and water vapor will be ejected through the rocket nozzle? How does
this compare with the original weight of hydrazine and nitrogen
tetroxide?
|