|
With fluorine, the roles of atoms in oxygen compounds are reversed.
All of the atoms through nitrogen were less electronegative than
oxygen, with the result that electron pairs were shifted toward
O or donated to O outright.
In contrast, fluorine is more electronegative and pulls electrons
toward itself, even in bonds with oxygen. Fluorine has several oxygen
compounds, which are chain molecules of the type F-O-F, F-O-O-F,
F-O-O-O-F, and so on. These are rare and unimportant, and do not
produce oxyacids, such as boric, carbonic, and nitric acids, when
added to water.
Fluorine is so electronegative that it does not make covalent bonds
with coordinating oxygen atoms in water, but steals electrons from
them instead to make fluoride ions. 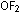 breaks up water molecules and releases
breaks up water molecules and releases 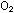 in the process of producing fluoride ions:
in the process of producing fluoride ions:
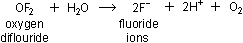
|
|
In writing formulas of binary compounds, the more electronegative
element is written second. Thus writing 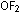 instead of
instead of 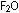 and naming the compound oxygen difluoride, rather than difluorine
oxide, is a reminder that in this compound fluorine is the more
electronegative of the two elements.
and naming the compound oxygen difluoride, rather than difluorine
oxide, is a reminder that in this compound fluorine is the more
electronegative of the two elements.
|