|
The nitrate ion is isoelectronic with the carbonate ion. If one
could reach into the nitrogen nucleus in the 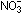 ion and "turn off" one proton, then
ion and "turn off" one proton, then 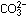 would result, with the same arrangement of atom 3 centers and electrons.
would result, with the same arrangement of atom 3 centers and electrons.
The extra nuclear charge on nitrogen causes it to hold the delocalized
electron more tightly, and discourages its binding a proton and
making the undissociated acid molecule. The nitrate ion has a weaker
attraction for protons than the carbonate ion, so nitric acid is
stronger than carbonic acid.
The lunar excursion module from the Apollo moon landings used another
oxide of nitrogen as part of its propellant. Its fuel was hydrazine
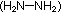 and methyl hydrazine
and methyl hydrazine 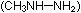 ,
and the oxidizer was dinitrogen tetroxide ,
and the oxidizer was dinitrogen tetroxide 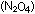 ,
a liquefied dimer of gaseous ,
a liquefied dimer of gaseous 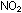 . .
Combustion produced mainly nitrogen and water, with a few other
oxides of nitrogen:
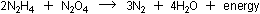
|
|
A big advantage for rocket design is that these two liquids are
hypergolic. This means that they ignite spontaneously when
mixed in the combustion chamber, making an ignition system for the
rocket motor unnecessary.
|
