|
We can regard a salt as the result of the neutralization of an acid
and a base. An acid dissolved in water releases hydrogen ions:
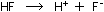
and a base dissolved in water produces hydroxide ions:
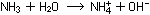
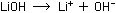
(Remember that all ions in aqueous solution are hydrated, or surrounded
by water molecules, even though this usually is not written explicitly.)
If we mix an acid and a base, a reaction occurs by the combination
of 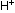 and
and 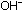 ions into water molecules:
ions into water molecules:
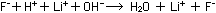
(Only a small percentage of the H-F molecules in solution will
be dissociated into 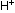 and
and 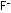 ions initially, therefore HF is called a weak acid. As the above
reaction occurs, however, more HF will dissociate.)
ions initially, therefore HF is called a weak acid. As the above
reaction occurs, however, more HF will dissociate.)
|
|
The real reaction is the combination of hydrogen and hydroxide ions;
the other ions only go along for the ride:
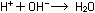
If the number of 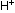 and
and 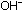 ions is the same, the final solution is neutral. The reaction of
an acid with a base in general is called neutralization, and represents
the "canceling out" of the acidic and basic properties
of the original solutions by the elimination of
ions is the same, the final solution is neutral. The reaction of
an acid with a base in general is called neutralization, and represents
the "canceling out" of the acidic and basic properties
of the original solutions by the elimination of 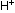 and
and 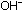 ions.
ions.
The neutralized solution shown on the next page is nothing but
an aqueous solution of equal quantities of  and
and 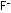 ions. If the solution is evaporated to dryness, salt crystals of
LiF are left behind. The result of acid-base neutralization is a
salt and water.
ions. If the solution is evaporated to dryness, salt crystals of
LiF are left behind. The result of acid-base neutralization is a
salt and water.
In principle, a fanatic chemist could season his beefsteak by pouring
over it equal quantities of lye or sodium hydroxide, NaOH, and hydrochloric
acid, HCl. The result after neutralization would be common table
salt, NaCl. If the chemist were very precise about quantities, and
very thorough about mixing, then he might get away with such a procedure,
but it is not recommended.
|