|
At the cathode,  ions accept electrons from the external circuit and become lithium
atoms, which plate out as a metal on the surface of the electrode:
ions accept electrons from the external circuit and become lithium
atoms, which plate out as a metal on the surface of the electrode:
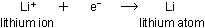
At the anode, 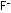 ions give up electrons to the external circuit and combine into
neutral
ions give up electrons to the external circuit and combine into
neutral 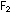 molecules, which bubble away as a gas:
molecules, which bubble away as a gas:

Such an arrangement for passing current through a molten salt is
called an electrolysis cell ("electro-lysis" meaning
"breaking down with electricity").
Electrolysis is one of the best ways of preparing pure metals such
as aluminum, which is electrolyzed commercially from a melt of aluminum
oxide ore.
|
|
In the context of the present discussion, electrolysis cells themselves
are not as important as is the essential idea that current in a
molten salt is carried by the migration of positive and negative
ions.
Current is carried in the same way in a solution of LiF or any other
salt in water: The hydrated positive and negative ions move in opposite
directions.
Salt crystals do not conduct electricity, because the ions are locked
in a crystalline lattice and can not move.
|
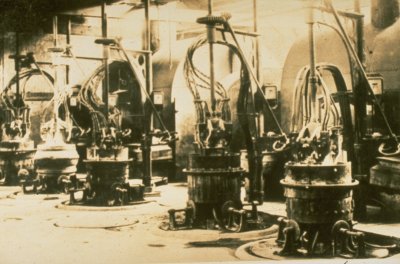
Hall-Heroult cells in early aluminium production
|
|
|