|
Apparatus
One 100cm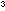 beaker.
beaker.
Three boiling tubes and rack.
Dropping pipette.
Chemicals
The quantities given are for one demonstration
Phenolphthalein indicator solution
(0.1g solid dissolved in 60cm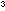 ethanol and 40cm
ethanol and 40cm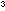 water)
water)
A few pellets of solid sodium hydroxide.
About 100cm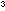 of 0.5 mol dm
of 0.5 mol dm 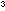 sodium hydroxide.
sodium hydroxide.
A few cm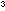 of approximately 2 mol dm
of approximately 2 mol dm 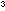 hydrochloric acid.
hydrochloric acid.
|
|
The Demonstration
Add a few drops of phenolphthalein solution to about 100cm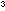 of 0.5 mol dm
of 0.5 mol dm 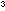 sodium hydroxide in a beaker until a deep pink colour is visible.
Divide this solution between the three boiling tubes. Leaving one
tube as a control, add hydrochloric acid dropwise to one of the
other two until the colour disappears. This is the 'usual' behaviour
of phenolphthalein. To the third tube, add two or three pellets
of solid sodium hydroxide and swirl to dissolve. The pink colour
will disappear in this tube too. The colour changes can be reversed
by appropriate additions of acid or alkali.
sodium hydroxide in a beaker until a deep pink colour is visible.
Divide this solution between the three boiling tubes. Leaving one
tube as a control, add hydrochloric acid dropwise to one of the
other two until the colour disappears. This is the 'usual' behaviour
of phenolphthalein. To the third tube, add two or three pellets
of solid sodium hydroxide and swirl to dissolve. The pink colour
will disappear in this tube too. The colour changes can be reversed
by appropriate additions of acid or alkali.
A white background will help.
The demonstration could be presented by starting with the colourless
solution of phenolphthalein in concentrated alkali and adding acid
to it to give an unexpected colour change.
|