|
Theory
Aldehydes such as glucose are reducing agents and will reduce
Ag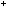 (aq) ions to metallic silver. They themselves are oxidised to carboxylate
ions. The reaction that occurs is:
(aq) ions to metallic silver. They themselves are oxidised to carboxylate
ions. The reaction that occurs is:
CH OH(CHOH) OH(CHOH) CHO
(aq) + 2Ag( CHO
(aq) + 2Ag(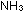 ) ) 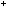 (aq) + 3OH
(aq) + 3OH (aq)
(aq) 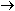
2Ag(s) + CH OH(CHOH) OH(CHOH) CO CO  (aq)
+ 4 (aq)
+ 4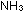 (aq)
+ 2H (aq)
+ 2H O(l) O(l)
Extensions
Try using an ordinary aldehyde instead of glucose, and show that
the reaction does not work with a ketone such as propanone.
|
|
Further Details
The silver can be removed from the silvering flask using concentrated
nitric acid. Work in a fume cupboard because nitrogen dioxide is
formed. There are reports of silvered flasks being kept for several
years as ornaments.
Safety
Wear eye protection.
There have been a few reports of alkaline ammonical silver nitrate
solutions exploding after standing for some time. This rare occurence
is thought to be caused by the formation of silver nitride or silver
fulminate. To avoid this risk, the ammonical silver nitrate solution
should not be made up before the demonstration and any silvering
solution left after the demonstration should not be placed in a
silver residues container but should be washed down the sink with
plenty of water. The silvered flask should be rinsed thoroughly
with water and the washings washed down the sink as soon as the
silvering has finished.
|