|
The demonstration
Place 150 cm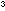 of the silver nitrate solution in a 250 cm
of the silver nitrate solution in a 250 cm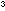 beaker and, working in a fume cupboard if possible, ass 880 ammonia
using a dropping pipette. A brown precipitate will form. Continue
to add the ammonia until the precipitate re-dissolves to give a
clear, colourless solution. Less than 5 cm
beaker and, working in a fume cupboard if possible, ass 880 ammonia
using a dropping pipette. A brown precipitate will form. Continue
to add the ammonia until the precipitate re-dissolves to give a
clear, colourless solution. Less than 5 cm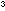 of ammonia will be needed. The solution then contains Ag(
of ammonia will be needed. The solution then contains Ag(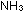 ) ) 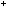 (aq).
(aq).
Add 75 cm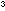 of the potassium hydroxide solution. A dark brown precipitate will
form. Add more ammonia dropwise until this precipitate redissolves
to give a clear, colourless solution. About 5 cm
of the potassium hydroxide solution. A dark brown precipitate will
form. Add more ammonia dropwise until this precipitate redissolves
to give a clear, colourless solution. About 5 cm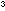 of ammonia will be needed.
of ammonia will be needed.
Pour this solution into the 1 dm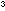 flask and add 12 cm
flask and add 12 cm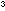 of the glucose solution. Stopper the flask and swirl the solution
so that the whole of the inner surface of the flask is wetted. The
solution will turn brown. Continue swirling until a mirror forms.
This will take about 2 minutes.
of the glucose solution. Stopper the flask and swirl the solution
so that the whole of the inner surface of the flask is wetted. The
solution will turn brown. Continue swirling until a mirror forms.
This will take about 2 minutes.
|
|
When a satisfactory mirror has formed, pour the solution down the
sink with plenty of water. Rinse out the flask well with water and
discard the washings down the sink. The flask can now be passed
around the class.
DO NOT SAVE THE SILVER SOLUTION IN A SILVER RESIDUE CONTAINER.
An alternative to plating the inside of the flask is to silver plate
the outside of a small glass objects which can be suspended in teh
plating solution by hanging them on threads. These objects must
be cleaned beforehand.
|