|
Some of this absorption causes breakdown to NO and atomic oxygen:

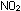
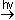 NO + O
NO + O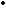
The NO can be reoxidized to 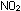 ,
and the oxygen atom goes on to do further damage. If you add the
two preceding reactions, you will see that the oxides of nitrogen
are really only the cyclic machinery for using photons of visible
light to tear oxygen molecules apart into atoms: ,
and the oxygen atom goes on to do further damage. If you add the
two preceding reactions, you will see that the oxides of nitrogen
are really only the cyclic machinery for using photons of visible
light to tear oxygen molecules apart into atoms:


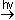 2O
2O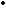
Free oxygen atoms are damaging because they combine with  to form ozone (
to form ozone (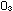 ): ):
  + O
+ O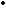
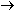
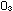
Ozone is an excellent oxidant that attacks organic matter, including
hydrocarbon pollutants, rubber, paint and plastics, and the lining
of our lungs.
|

|
