|
The primary offenders in the photochemical smog that blankets the
Los Angeles basin and covers an increasing number of other cities
on bad days are unburned hydrocarbons and oxides of nitrogen. Even
with stringent controls on stationary sources of pollution, as in
Los Angeles, the automobile remains a serious polluter.
Smog from oxides of nitrogen begins with nitric oxide, NO, which
is formed at high temperatures from  and
and 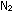 in
the combustion chambers of automotive engines: in
the combustion chambers of automotive engines:
  +
+ 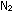 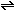 2NO
2NO
NO is emitted in the exhaust and is oxidized by atmospheric  to brown nitrogen dioxide:
to brown nitrogen dioxide:
 NO +
1/2 NO +
1/2  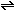
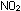
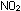 is brown because
its unpaired electron leads to closely spaced electronic energy
levels that permit absorption of light in almost the entire visible
spectrum. is brown because
its unpaired electron leads to closely spaced electronic energy
levels that permit absorption of light in almost the entire visible
spectrum.
|

|
