|
After the forward and reverse reactions proceed for a suitable length
of time, the concentrations of A and B will settle down to a fixed
ratio, which will not depend on the starting conditions or the absolute
number of A and B molecules present. This is the condition of equilibrium,
at which forward and reverse rates are exactly in balance:
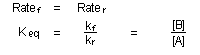
Equilibrium does not imply that all chemical activity has stopped,
only that forward and reverse reactions are proceeding at the same
rate, so no further net change in the amounts of reactants and products
occurs.
|
|