|
11.
If the U. S. government had allowed helium to be exported, how many
moles of helium would have been needed to fill the Hindenberg? How
much weight could the ship then have lifted, using He instead of
 ? ?
12. Why are  and He the only two gases that can be used in lighter-than-air craft?
and He the only two gases that can be used in lighter-than-air craft?
13. If the gas bags were filled on a hot day, at 30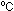 ,
by what percent would they have shrunk if the temperature fell to
15 ,
by what percent would they have shrunk if the temperature fell to
15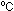 ? ?
14. If  , ,
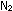 ,
and ,
and 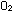 all behave as ideal gases, would the lifting ratio of a balloon
(the number of times the weight of gas it can lift) change as the
temperature changed? Why, or why not?
all behave as ideal gases, would the lifting ratio of a balloon
(the number of times the weight of gas it can lift) change as the
temperature changed? Why, or why not?
15. When 1 g of unknown liquid is vaporized at 80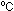 and 1.01 atm pressure, it occupies a volume of 0.622 liter. How
many moles of the liquid are present?
and 1.01 atm pressure, it occupies a volume of 0.622 liter. How
many moles of the liquid are present?
16. What is the molecular weight of the liquid of Problem
15?
17. If the molecules of the liquid in Problem 15 are 34.7%
oxygen by weight, and contain only carbon, hydrogen, and oxygen,
what might the liquid be? (We have encountered it previously in
this problem set.)
|
|