|
Example.
An eight-foot diameter weather balloon is filled with 7600 liters
of  gas
at 1 atm. pressure and 25 gas
at 1 atm. pressure and 25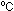 .
As the balloon rises to an altitude where the pressure is only 0.70
atm, the temperature drops to -20 .
As the balloon rises to an altitude where the pressure is only 0.70
atm, the temperature drops to -20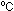 .
What then is the volume of the balloon? .
What then is the volume of the balloon?
Solution. Let sea-level conditions be denoted by subscript
1, and high altitude conditions, by 2. The number of moles of gas
does not change, so we can use the ideal gas law in the form
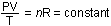
or
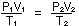
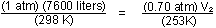
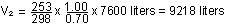
The decrease in pressure to 0.70 atm causes an increase in volume
by a factor of 1.00/0.70, but the simultaneous drop in temperature
causes a shrinkage by a factor of 253/298. The balloon does not
expand as much as it would have if the temperature had remained
constant.
|

|
