|
Example.
An eight-foot diameter weather balloon is filled with 7600 liters
of  at
1atm pressure and 25 at
1atm pressure and 25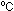 .
How many moles of hydrogen gas are present? .
How many moles of hydrogen gas are present?
Solution. P = 1 atm, V = 7600 liters, T = 25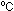 = 298K.
= 298K.
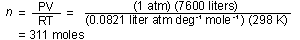
As an interesting sidelight, we can calculate the lifting power
of the balloon.
Solution. The 311 moles of  gas weigh 311 moles X 2.016 g
gas weigh 311 moles X 2.016 g 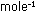 = 627 g. The lifting power of the balloon is the difference between
this and the weight of the air that the balloon displaces.
= 627 g. The lifting power of the balloon is the difference between
this and the weight of the air that the balloon displaces.
The same volume of air also would contain 311 moles (Avogadro's
principle), and air can be considered a mixture of 80% nitrogen
gas and 20% oxygen gas.
|
|
The average molecular
weight of the air mixture then is
80 % x 28.013 g 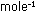 + 20 % x 32.000 g
+ 20 % x 32.000 g 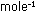
=28.81 g 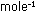
The weight of air displaced is
311 moles x 28.81 g 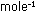 = 8960 g
= 8960 g
The buoyancy of the balloon is the difference in weight of air and
hydrogen:
8960 g - 627 g = 8333 g
The balloon therefore can lift slightly more than 8 kilograms, or
18 pounds, of payload. We now can take into account the simultaneous
change of pressure and temperature, and correct the flaw in the
weather balloon example first used to illustrate Boyle's law.
|