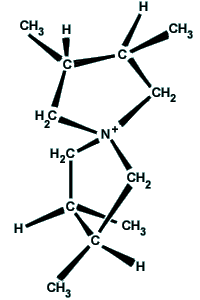
A chiral molecule (from
the Greek word Chiros - 'hand') is a molecule that is distinguishable
from, and not superposable onto, its mirror image in the same way that
left and right hands are distinguishable. Chiral molecules are optically
active and rotate plane-polarized light. A chiral molecule and its
mirror image partner are called enantiomers (from the Greek word
for 'both'). |
||
| The group-theoretical criterion of whether
or not a molecule is superimposable on its mirror image is that it should
not have an improper-rotation axis Sn. However,
we must be alert for improper-rotation axes that are present in disguise.
From our definitions, we know that the S1 axis is
identical to a mirror plane |
||
|