Substituting in the rate equation, we get
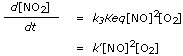 |
in which k� = k3Keq.
This is the same rate expression that would have resulted if the reaction had occurred by the simultaneous three-molecule collision of N0, N0, and 02, but the mechanism just proposed assumes a series of two-molecule collisions instead. One cannot decide the actual mechanism of a reaction from the rate equation alone.
For this example, it would be necessary to carry out chemical experiments to determine the presence or absence of reaction intermediates such as N202. Finding them would support the proposed mechanism; but not finding them might only mean that the chemical detection methods were not sensitive enough.
This is why the number of proposed reaction mechanisms in the chemical literature is much greater than the number of well-established mechanisms.