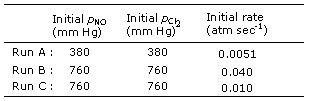|

|
| 1. The
half-life of l4C is 5570 years. |
| i) What is the
first-order
Rate
constant, k. |
|
|
|
ii) How many radioactive disintegrations will occur
per minute in a one-gram sample of pure 14C?
|
|
|
| iii) A fresh sample of
wood shows 15.3 disintegrations per gram of carbon. 1.50x10-10%of
its carbon content is therefore 14C. A sample
of wood from an Egyptian mummy case gives 9.4 decay counts per minute per
gram of carbon. How old is the mummy case? |
|
|
|
2. For the reaction 2NO + H2
---> N2O + H2O
it was found in a series of experiments that doubling the initial concentration
of NO made the initial reaction rate four times as fast, whereas doubling
the initial H2 concentration only made the initial
reaction twice as rapid. What is the rate
law for this reaction?
Rate=:
|
|
|
|
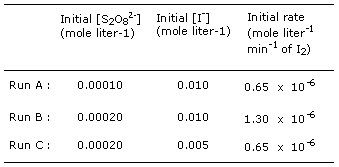
3. The method of studying the order
of a reaction as outlined in the preceding problem is known as the initial-rate
method. The reaction S2O82-
+ 2I- --> 2SO42-
+ I2 in aqueous solution was studied by the
initial-rate method, with the results for three trial runs given below.
What is the rate
expression for this reaction and what is the overall order
(n) of the reaction? |
|
|
|
4. In the gas-phase reaction 2NO + Cl2
--> 2NOCl, the results given below were obtained in three runs of an
initial-rate experiment.
What is the correct rate
expression and order
(n) for the reaction? |
|
|
5. Tert-butyl bromide reacts with
water to form tert-butyl alcohol and HBr by the overall reaction
(CH3)3CBr + H2O
--> (CH3)3COH + HBr.
i) What would be the rate
expression if the reaction were a simple bimolecular collision? Rate
= |
|
|
ii) What would the rate expression
be if the
mechanism were
| (CH3)3CBr
--> (CH3)3C+
+ Br- |
(slow) |
| (CH3)3C+
+ H2O --> (CH3)3COH
+ H+ |
(fast) |
|
|
|
| iii) Could you distinguish between
the two mechanisms in (i) & (ii) if the reaction takes place in dilute
aqueous solution? |
|
|
6. The following data
were measured for the decomposition of ammonia, 2NH3
--> N2 + 3H2
| Time (sec) |
0 |
1 |
2 |
| [NH3] (mole liter-1) |
2.000 |
1.993 |
1.987 |
Plot the logarithms of the concentrations against
time and show that this is a first-order
process.
i) What is the rate
constant? |
|
|
|
ii) What is the half-life
for ammonia decomposition?
|
|
|
7. The reaction SO2Cl2
--> SO2 + Cl2 is a
first-order
process with a rate constant of k = 2.2x10-5 sec-1
at 320�C. What fraction of the initial SO2Cl2
will have decomposed after heating at 320�C for 90 minutes?
|
|
|
| 8. For the decomposition of N205
in carbon tetrachloride, a plot of ln [N205]
against time gives a straight line. The rate constant is k = 6.2
x 10-4 sec at 45�C. If one begins with 1 mole
of N205 in a 1-liter
flask, how long will it take for 20% of the N205
to decompose and what is the half-life (t1/2)of
this decomposition? |
|
|
|
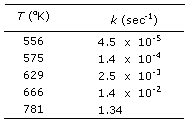
9. The following data give the temperature dependence
of the rate
constant for the decomposition of N205:
Make an Arrhenius
plot. From the graph what is the energy of activation for the reaction?
|
|
|
10. For a reaction with an activation
energy of 4.0 kcal mole-1 calculate the fraction
of molecules with energy Ea, or greater,
f = e- Ea/RT, for 0�K, 100�K, 1000�K,
10,000�K, and 100,000�K?. Now plot these values on a linear graph of f
versus T.
What does this graph tell you about the influence of temperature on reaction
rate? |
|
|
11. The rate
constant for the reaction H2 +I2
--> 2HI was measured at several temperatures, with the following
results:
What is the energy
of activation for the reaction? |
|
|
| 12. Using the Ea
for the reaction H2 +I2 -->
2HI calculated in the previous problem, and standard heat
of formation data from the Appendix
what is the activation energy for the reverse reaction, 2HI --> H2
+I2? |
<
|
|
| 13. It is often said that, near room
temperature, the rate of a reaction will double if the temperature is increased
by 10�C. What is the activation energy, Ea, of
a reaction whose rate exactly doubles between 27�C and 37�C? |
|
|
| 14. What is the activation energy,
Ea, for a reaction whose rate is tripled
by a temperature increase from 20�C to 30�C? |
|
|
15. For the decomposition of CH3I
at 285�K, Ea, is 43 kcal mole-1.
i) What fraction of the molecules have this energy or greater at 285�K?
|
|
|
| ii) What is the percentage increase
in the fraction of molecules with energy greater than Ea
when the temperature is raised to 300�K. (Assume that Ea
is not a function of temperature - which is nearly so but not quite). |
|
|
| 16. The rate constant for the decomposition
of N205 in carbon tetrachloride
is 6.2 x 10 4 sec-1 at 45�C. In light of this
information what is the rate
constant at 200�C, if the activation
energy is 24.7 kcal mole-1? |
|
|
![]()