In a series of reactions, the slowest one has the greatest influence on reaction rates. To invoke a painful analogy, if it takes ten days to to get a certified letter from Los Angeles to the White House, then rushing to get it into the one o'clock mail instead of the three o'clock will make little difference in the long run.
Formation of HBr
The rather horrendous rate law for the HBr reaction,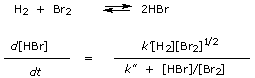 |
arises because the true process is a chain reaction that involves first the dissociation of Br2 molecules into atoms, then the reaction of atoms with other H2 and Br2 molecules:
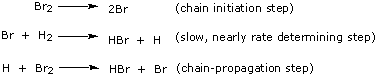 |