|
Oxidation does not necessarily
require the outright removal of electrons, as we have said.
Oxidation-reduction reactions in which electrons are actually
moved from one substance to another are especially useful,
since if the donor and recipient can be isolated, and the
electrons made to flow through an external wire or circuit,
some of the free energy of the oxidation-reduction process
can be harnessed to do useful
work. As an example, zinc metal has less of an affinity for
its 4 outer electrons than metallic copper does. In a competition
between Cu2+ and Zn2+ ions for electrons,
copper ions will win. The reaction
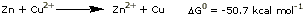
is highly spontaneous,
with a standard free energy change of -50.7 kcal per mol.
If we dip a zinc strip into a copper sulfate solution, as
shown opposite, the zinc will be eaten away, a spongy layer
of metallic copper will plate out on the zinc strip, and the
deep blue color of copper sulfate will gradually fade. (Zinc
sulfate, which is formed, is colorless.) In contrast, if we
immerse a copper strip in a zinc sulfate solution, no reaction
will occur because the reverse reaction is highly nonspontaneous,
with a +50.7 kcal per mol free energy
barrier to surmount.
This spontaneous transfer of electrons from zinc to copper
is not useful because the free energy released is dissipated
as heat. It is analogous to burning a spoonful of sugar with
a match instead of eating it and converting the free energy
of oxidation into useful muscle work. If some means could
be found to separate the removal of electrons from zinc (oxidation)
from the donation of electrons to copper ions (reduction),
then the electrons might be made to do something useful along
the way.
|
|