|
However, one need only select one substance as a reference
standard, and tabulate the free energies or voltages
for reactions of all other substances with this standard.
The free energy of a cell not involving the standard
substance then can be found by subtracting one of these
reactions from another, and subtracting free energies
in the same way. The contribution of the
"standard" reaction cancels out.
The standard half-reaction that has been chosen is
the oxidation of hydrogen gas to hydrogen ions in solution:
Pairing the zinc, nickel, and copper half-reactions
separately with this half-reaction leads to the following
tabulation:
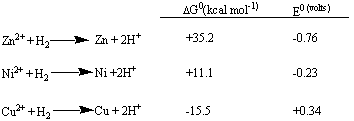
|