|
In the cells considered so far, it has been implied
that the electrodes were in contact with ions in solution.
The dry cell, shown on the right, is especially convenient
because the solutions
are replaced by a moist paste
in a sealed container. The zinc
casing is the anode,
with the half-reaction

The cathode
is a central carbon rod
surrounded by a paste of manganese
dioxide (MnO2), ammonium
chloride (NH4Cl), and water.
The paste and the zinc casing are separated only by
a porous
paper barrier. The cathode reaction is a complex
one, but can be represented as
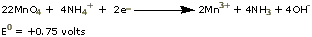
A dry cell delivers about
1.5 volts (0.76V + 0.75V). If the cell is used continuously,
the current slowly decreases as ammonia gas builds up
around the carbon rod and insulates it. If the cell
is allowed to rest, this ammonia diffuses
toward the anode and combines
with zinc ions to form a complex ion, Zn(NH3)42+
. The cell then is able to deliver a stronger current
again. This is why flashlight batteries appear to run
down with steady use, but to recover after standing
idle for a time.
|