|
Free energies of balanced
chemical reactions always are additive.
Using only the information that we have so far about
Zn-Cu and Ni-Cu cells, we can predict what would happen
if we built a Zn-Ni cell. The reaction of this Zn-Ni
cell can be obtained by subtracting
the Ni-Cu reaction from
the Zn-Cu reaction. (Remember
that subtracting a reaction is the same as adding the
reverse reaction, with the opposite sign for the free
energy.)
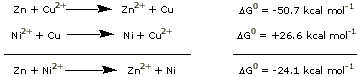
The negative free energy of the Zn-Ni reaction means
that it is spontaneous
in the direction written. The potential
of the Zn-Ni cell can be calculated from D
G0 =-nFE0
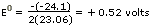
The positive cell potential
means the same thing as the negative
free
energy change: The cell reaction in the direction
written is spontaneous.
|