|
Phosphorus
is as essential for life as is nitrogen, but for a different reason.
It is absent in proteins, but present in the backbones of nucleic
acid chains such as DNA. Even more importantly, phospborus is at
the heart of the central energy-storage molecule, adenosine triphosphate
(ATP), shown at the left. Just as silicates can form polysificate
chains by sharing comer oxygen atoms of silicate tetrahedra, so
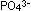 can polymerize
into polyphosphates. Two linked tetrahedra build a pyrophosphate
ion, can polymerize
into polyphosphates. Two linked tetrahedra build a pyrophosphate
ion, 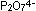 . Metaphosphates
have rings or long chains of . Metaphosphates
have rings or long chains of 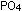 tetrahedra. ATP consists of three linked phosphate tetrahedra, with
ribose (a sugar ring) and adenine (a nitrogen-containing base) hooked
onto one end. ATP is remarkable for the large amount of energy it
gives off when one of the phosphate groups is hydrolyzed away by
water:
tetrahedra. ATP consists of three linked phosphate tetrahedra, with
ribose (a sugar ring) and adenine (a nitrogen-containing base) hooked
onto one end. ATP is remarkable for the large amount of energy it
gives off when one of the phosphate groups is hydrolyzed away by
water:

ADP is an abbreviation for adenosine diphosphate, which has one
phosphate tetrahedron removed from ATP. Most other hydrolysis reactions
yield only two or three kilocalories of energy. When ATP is synthesized
from ADP and inorganic phosphate, an unusually large amount of energy
can be stored in the final phosphate bond, which then is available
for later use:
ADP + phosphate + 7.3 kcal of energy  ATP +
ATP + 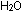
|
|