|
In all of these biological
nitrogen-containing molecules, nitrogen is in a reduced state with
an oxidation number of -3, as in ammonia. Living organisms require
a supply of reduced nitrogen to synthesize these molecules, but
most of them cannot get it from atmospheric 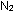 .
Fortunately, there are bacteria that can "fix" nitrogen by converting .
Fortunately, there are bacteria that can "fix" nitrogen by converting
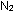 to to 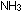 .
Energy is required to accomplish this, but this expenditure of energy
is worthwhile to the bacterium because it provides a source of reduced
nitrogen for synthetic purposes. When organisms die, most of the
reduced nitrogen in proteins and other compounds remains reduced
and is reused by other organisms. However, there are inevitable
losses in this process, and new reduced nitrogen is required. The
rest of the living world cannibalizes the efforts of nitrogen-fixing
bacteria to keep the system going. Many such bacteria live in the
root nodules of legumes such as soybeans, which is why agricultural
land sometimes is replenished by growing a crop of soybeans and
plowing the crop under at maturity. If given nitrates (with nitrogen
ON = +5) instead of ammonia (with nitrogen ON = -3), plants can
reduce them and incorporate them into protein. Thus the critical
factor is not the oxidation state of nitrogen, it is the unreactivity
of gaseous .
Energy is required to accomplish this, but this expenditure of energy
is worthwhile to the bacterium because it provides a source of reduced
nitrogen for synthetic purposes. When organisms die, most of the
reduced nitrogen in proteins and other compounds remains reduced
and is reused by other organisms. However, there are inevitable
losses in this process, and new reduced nitrogen is required. The
rest of the living world cannibalizes the efforts of nitrogen-fixing
bacteria to keep the system going. Many such bacteria live in the
root nodules of legumes such as soybeans, which is why agricultural
land sometimes is replenished by growing a crop of soybeans and
plowing the crop under at maturity. If given nitrates (with nitrogen
ON = +5) instead of ammonia (with nitrogen ON = -3), plants can
reduce them and incorporate them into protein. Thus the critical
factor is not the oxidation state of nitrogen, it is the unreactivity
of gaseous 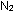 and
the inability of plants or animals to do anything with it. and
the inability of plants or animals to do anything with it.
|
|
The triple bond in the
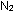 molecule is so
stable and resistant to attack that reactions involving molecule is so
stable and resistant to attack that reactions involving 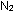 ,
either oxidation or reduction, are hopelessly slow. Nitrogen-fixing
bacteria, alone among living organisms, have catalytic enzymes that
speed up these reactions. ,
either oxidation or reduction, are hopelessly slow. Nitrogen-fixing
bacteria, alone among living organisms, have catalytic enzymes that
speed up these reactions.
A limited amount of 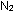 is fixed in the atmosphere each year by electrical discharge in
lightning, whereby the relative unreactivity of
is fixed in the atmosphere each year by electrical discharge in
lightning, whereby the relative unreactivity of 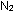 is overcome by energy from the lightning discharge. The products
include acidic oxides of nitrogen, which means that the rain during
a thunderstorm actually is a very dilute solution of nitric acid.
More important as a rival to bacterial processes is industrial nitrogen
fixation by methods involving catalysts and high pressures that
were developed by Fritz Haber in Germany during World War I:
is overcome by energy from the lightning discharge. The products
include acidic oxides of nitrogen, which means that the rain during
a thunderstorm actually is a very dilute solution of nitric acid.
More important as a rival to bacterial processes is industrial nitrogen
fixation by methods involving catalysts and high pressures that
were developed by Fritz Haber in Germany during World War I:

Once fixed as ammonia, nitrogen then can be used directly as fertilizers
to grow crops, or oxidized to nitrates for explosives to blow them
up.
|