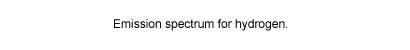|
Absorptions or emissions involving the same lower quantum state
belong to the same series because their spectral lines appear near
one another.
The spacings between quantum levels in hydrogen are so great that
all lines of the 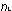 = 1 series fall in the high-energy ultraviolet region.
= 1 series fall in the high-energy ultraviolet region.
Most of the 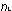 = 2 series of absorption lines fall in the visible region, and the
= 2 series of absorption lines fall in the visible region, and the
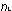 = 3 lines are found in the near infrared region.
= 3 lines are found in the near infrared region.
The actual visible spectrum of the hydrogen atom is shown to the
right. This spectrum, and its exact match with that predicted by
the Bohr theory at a time when no other explanation of atomic spectra
was available, was convincing evidence that Bohr was on the right
track.
|

|