|
For atomic hydrogen, the lowest-energy state, with the 0.53 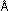 radius orbit, has an energy of
radius orbit, has an energy of  = - 313 kcal
= - 313 kcal 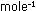 .
Atomic hydrogen in this state is 313 kcal .
Atomic hydrogen in this state is 313 kcal 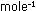 more stable than an ionized atom. This 313 kcal
more stable than an ionized atom. This 313 kcal 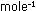 is the ionization energy of atomic hydrogen. Although the numerical
value is calculated from first principles in the Bohr theory (using
quantities such as Planck's constant and the mass and charge of
the electron), it agrees exactly with the measured value of the
ionization energy of hydrogen given in Chapter 3. This is the kind
of agreement that builds confidence in any theory.
is the ionization energy of atomic hydrogen. Although the numerical
value is calculated from first principles in the Bohr theory (using
quantities such as Planck's constant and the mass and charge of
the electron), it agrees exactly with the measured value of the
ionization energy of hydrogen given in Chapter 3. This is the kind
of agreement that builds confidence in any theory.
In the n = 2 quantum state, atomic hydrogen has an energy of
 
This is a higher (less negative) energy than the n = 1 state. In
this state, the atom is only 78 kcal 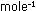 more stable than the ion. The higher the quantum number, n, the
closer the energy comes to that of the ionized atom. Ionization,
or removal of the electron, can be considered as placing the electron
in quantum state n =
more stable than the ion. The higher the quantum number, n, the
closer the energy comes to that of the ionized atom. Ionization,
or removal of the electron, can be considered as placing the electron
in quantum state n =  (i.e., the electron is an infinite distance from the nucleus), with
energy
(i.e., the electron is an infinite distance from the nucleus), with
energy
 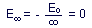
|
|