|
Max Planck first proposed this relationship between energy and frequency
in 1900 as part of his study of the way in which heated solids emit
radiation. The constant h is called Planck's constant in his honor.
It was Albert Einstein who successfully explained the photoelectric
effect. He showed that Planck's constant also was applicable to
the photoelectric effect, and appeared to be a universal constant
relating energy to frequency of radiation.
Here the paradox arose.
How could light simultaneously be a wave with frequency 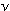 ,
and a collection of particles with individual energies E? ,
and a collection of particles with individual energies E?
How could a wave act like particles, or particles simulate a wave?
Was light really a wave, or a stream of particles?
|
|