|
9.
What is meant by s and p
symmetry in molecular orbitals? How is this nomenclature an obvious
extension of the s and p notation for atomic orbitals? Explain in
terms of 180 rotations and the signs of the wave functions.
rotations and the signs of the wave functions.
10. How is the bond energy of an electron-pair bond related
relative energies of the bonding and antibonding MO's and the AO's
from which they were derived? Explain with a diagram. If the energy
difference between the AO's and the bonding MO in H is x kcal mole
is x kcal mole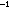 ,
what is the bond energy of H ,
what is the bond energy of H ? ?
11. What is the relative order of increasing energies for
the six MO's derived from the six outer p AO's on tw o atoms in
a diatomic molecule? How does this energy sequence account for the
observed bond orders in the diatomic molecules obtained from second-row
elements?
12. For which of the second-row elements do diatomic molecules
not exist? Why, in terms of MO theory? For which of these elements
do diatomic molecules occur only at high temperatures? What is the
their state at 298K, and why? For which of these elements is the
diatomic molecule the stable form at room temperature?
13. Which of the diatomic molecules of the second-row elements
have unpaired electrons? How many unpaired electrons do they have?
Why are these electrons not paired in the same orbital? How does
the Lewis electron-dot model account for these unpaired electrons?
|
|