|
The
loss of the infrared is of no consequence, because these wavelengths
contain so little energy per photon that they are not very good
as energy sources. At the short wavelength end of the solar spectrum,
it is fortunate for us that everything shorter than 2900 Å
is cut off by the ozone layer, be cause these "hard ultraviolet"
photons are energetic enough to break the C-C and C-N single bonds
that hold proteins and other biological macromolecules together.
The energy of a C-C single bond, 83 kcal mole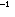 ,
corresponds to a wavelength of 3500 Å. These and shorter wavelengths
are potentially lethal, which is why ultraviolet lamps can be used
to kill bacteria and other microorganisms. Life evolved in the oceans,
bathed in a "window" of electromagnetic radiation from about 2900
Å to 8000 Å, with maximum abundance around 5000 Å.
It is no accident that living creatures developed means of using
this radiation as an energy source, and as a means of sensing the
environment through vision. The molecules that absorb energy in
this wavelength range are the aromatic and linear conjugated molecules
with delocalized double bonds. We will see in subsequent chapters
how the chlorophylls and carotenoids in photosynthesis, and retinal
in vision, all use the light-absorbing properties of delocalized
p molecular orbitals. ,
corresponds to a wavelength of 3500 Å. These and shorter wavelengths
are potentially lethal, which is why ultraviolet lamps can be used
to kill bacteria and other microorganisms. Life evolved in the oceans,
bathed in a "window" of electromagnetic radiation from about 2900
Å to 8000 Å, with maximum abundance around 5000 Å.
It is no accident that living creatures developed means of using
this radiation as an energy source, and as a means of sensing the
environment through vision. The molecules that absorb energy in
this wavelength range are the aromatic and linear conjugated molecules
with delocalized double bonds. We will see in subsequent chapters
how the chlorophylls and carotenoids in photosynthesis, and retinal
in vision, all use the light-absorbing properties of delocalized
p molecular orbitals.
|
|