|
Any
attached side group on the ring that increases the number of atoms
in the delocalized system will shift the electronic absorption toward
lower energy and longer wavelengths. Early industrial dyestuff chemists
knew by experience that some chemical groups such as -OH and -NH would shift the colors of aromatic molecules down the series yellow-orange-red-purple-blue-green,
the complement of the absorbed visible spectrum, violet-blue-green-yellow-orange-red,
before they understood the relationship among color, frequency,
and energy. Adding an -OH group to benzene to produce phenol, C
would shift the colors of aromatic molecules down the series yellow-orange-red-purple-blue-green,
the complement of the absorbed visible spectrum, violet-blue-green-yellow-orange-red,
before they understood the relationship among color, frequency,
and energy. Adding an -OH group to benzene to produce phenol, C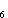 H H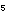 OH,
shifts the center of the absorption band slightly, from 2550 Å
to 2700 Å, since the oxygen in the -OH group is rich in electrons.
Phenol is an acid, and can dissociate and lose the proton of the
-OH to form the phenolate ion: OH,
shifts the center of the absorption band slightly, from 2550 Å
to 2700 Å, since the oxygen in the -OH group is rich in electrons.
Phenol is an acid, and can dissociate and lose the proton of the
-OH to form the phenolate ion:
C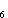 H H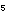 OH
--> C OH
--> C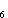 H H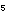 O O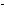 + H+
+ H+
phenol phenolate
ion
|
|