|
The Sp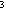 hybridization model also can be used for molecules involving N and
O, with lone electron pairs filling some of the Sp
hybridization model also can be used for molecules involving N and
O, with lone electron pairs filling some of the Sp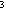 orbitals. Thus an improved picture of water (below) employs two
of the Sp
orbitals. Thus an improved picture of water (below) employs two
of the Sp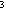 orbitals in bonds with hydrogen, and the other two for the two lone
electron pairs on 0. This Sp
orbitals in bonds with hydrogen, and the other two for the two lone
electron pairs on 0. This Sp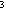 model predicts a tetrahedral H-0-H bond angle of 109.51. The smaller
observed angle of 105
model predicts a tetrahedral H-0-H bond angle of 109.51. The smaller
observed angle of 105 can be explained by the strong repulsion generated by the lone pairs,
which are closer to the oxygen atom and to each other than are the
bonding electron pairs.
can be explained by the strong repulsion generated by the lone pairs,
which are closer to the oxygen atom and to each other than are the
bonding electron pairs.
|
|