|
The
Lewis diagram of a water molecule, at the bottom left, still holds,
but MO theory identifies the two oxygen lone pairs explicitly as
being in the 2s and 2p. orbitals. However, something is badly wrong
with the simple localized MO theory as it has been presented here,
because it predicts that the H-O- H bond angle will be 90 |
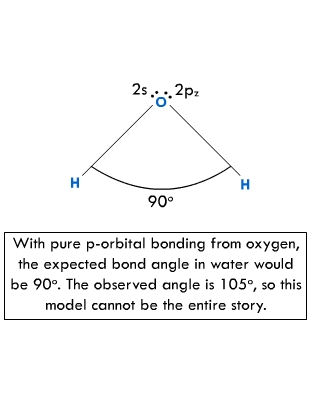
|