|
The
bond line represents the bonding 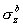 orbital
electrons, and the three lone pairs around F are in the 2s, 2p orbital
electrons, and the three lone pairs around F are in the 2s, 2p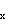 and 2p
and 2p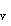 orbitals. This treatment of HF illustrates how two different quantum
levels on different atoms can be combined if they have similar energies.
It also illustrates the effect of difference in electronegativities
on the character of the chemical bond. The bonding MO in HF is closer
in energy to the 2p
orbitals. This treatment of HF illustrates how two different quantum
levels on different atoms can be combined if they have similar energies.
It also illustrates the effect of difference in electronegativities
on the character of the chemical bond. The bonding MO in HF is closer
in energy to the 2p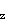 AO of fluorine from which it came, and less like the 1s AO of hydrogen.
The antibonding MO, in contrast, is closer in energy to the hydrogen
1s. If you worked out the mathematics of combining AO's into MO's,
you would find that the bonding
AO of fluorine from which it came, and less like the 1s AO of hydrogen.
The antibonding MO, in contrast, is closer in energy to the hydrogen
1s. If you worked out the mathematics of combining AO's into MO's,
you would find that the bonding 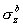 MO
has a greater contribution from the fluorine 2p MO
has a greater contribution from the fluorine 2p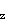 and the antibonding MO is more like the hydrogen 1s orbital. In
H
and the antibonding MO is more like the hydrogen 1s orbital. In
H , of course,
the two atoms are identical, and the bonding and antibonding MO's
have equal contributions from both atoms. , of course,
the two atoms are identical, and the bonding and antibonding MO's
have equal contributions from both atoms.
The lower energy of the
fluorine 2p AO, compared with that of the hydrogen 1s, is a reflection
of the fact that F is more electronegative than H, and holds onto
its outer electrons more tightly. In general, when two atoms of
different electronegativities are combined, the bonding MO's are
more like the AO's of the more electronegative element in both shape
and energy, and the antibonding MO's resemble those of the less
electronegative atom.
|
|